For this first Paper Digest, I’ve picked two papers. The first one is from Steffen Lemke’s group and focuses on the cephalic furrow (CF) - a structure that forms at the head-trunk border in Drosophila embryo as the gastrulation begins. It was published back-to-back with another study on the same topic in the same Nature issue. Personally, I preferred Lemke’s paper, since it leans more toward genetics than biomechanics. That’s the one I had planned for my IRL journal club, which I unfortunately had to cancel due to personal reasons. I’ll skip the Vellutini et al. paper here, but I’ll include links to both below.
Paper 1: Patterned invagination prevents mechanical instability during gastrulation.
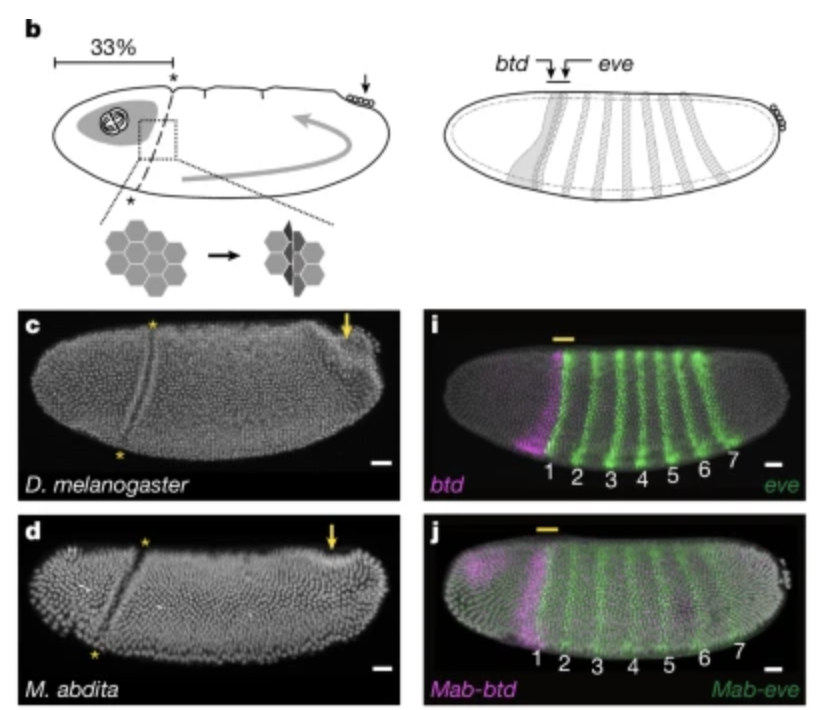
The authors showed that the CF forms only in Cyclorrhaphan flies (those include Drosophila) and that it helps mitigate the stress generated by collisions of dividing head and trunk regions. Disruption of CF formation, e.g. by a specific eve mutant that removes only the first eve stripe, leads to tissue buckling and developmental defects that persist well into later embryogenesis.
Non-Cyclorrhaphan flies, like Chironomus riparius deal with this issue in a different way. Instead of forming the CF, they utilize an out-of-plane mitosis which reduces the mechanical stress.
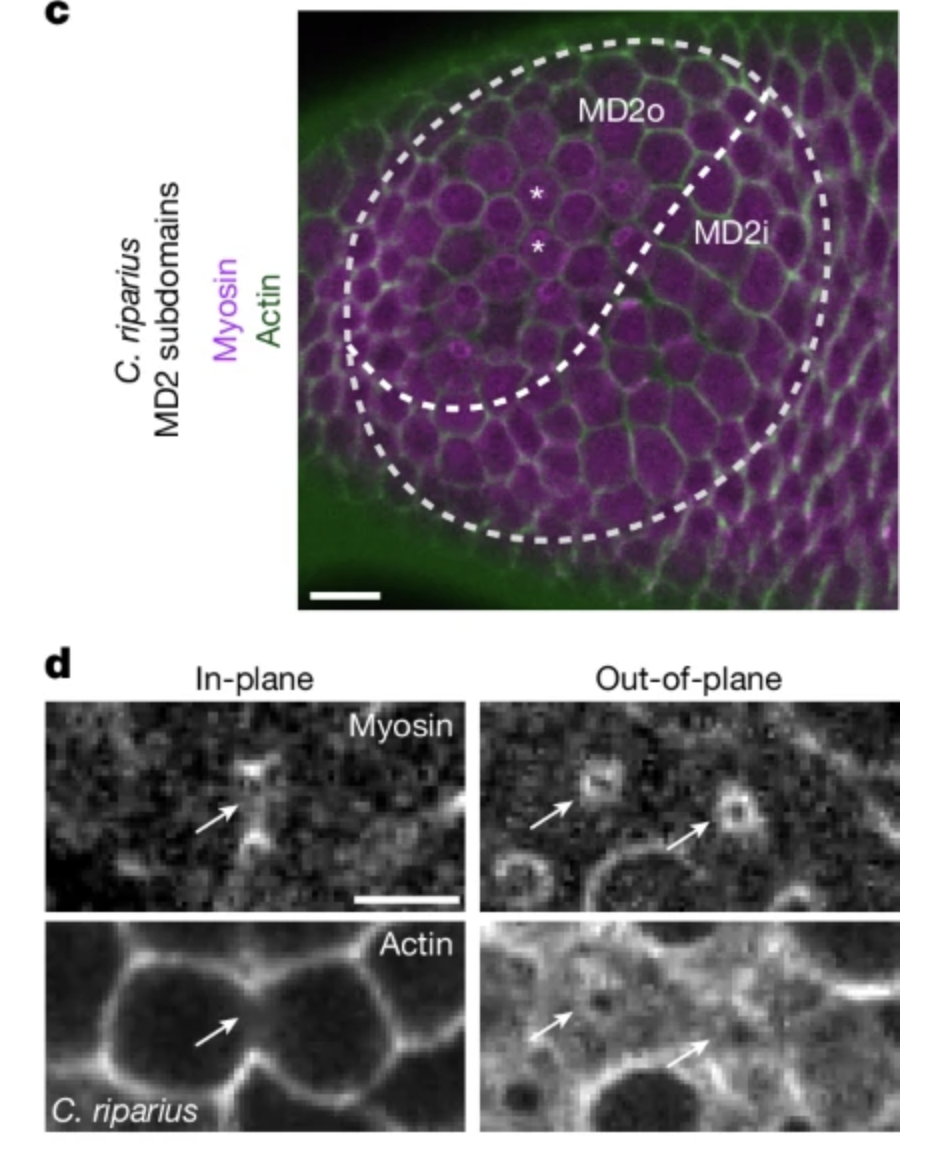
To test their model, the authors introduced out-of-plane mitoses into Drosophila flies mutants that don’t form CF and witnessed that it created a sink for mechanical stress and reduced the severity the effect of CF loss, e.g. half of the flies didn’t show any buckling (see Figure 6).
I think this is a solid and comprehensive study that consistently supports its proposed model. It was under review for almost two years (phew!), so congratulations to the authors. My favorite CF fact - told to me by a colleague - is that it has nothing to do with head formation and disappears well before the embryo’s head starts to properly develop. Its formation is “just” a way to relieve the stress generated by rapid cell division in the spatially confined embryo.
Paper 2: One mother for two species via obligate cross-species cloning in ants.
This one is mind blowing. It’s so bizarre, it even inspired Randall Munroe to make this xkcd:
The paper reads like a thrilling detective story: there’s a mystery, an investigation and a solution. It’s short, elegant, and so good that I’m including all three figures here.
It starts with two European harvester ants species: Messor ibericus and Messor structor. I know almost nothing about ants beyond school-level knowledge, but I’ve read that there are three castes for these two species:
Queens (egg-laying females without wings)
Males (winged, haploid, fertile, but short-lived)
Workers (wingless, diploid, and sterile).
First weird observation is that M. ibericus queens produce workers by mating with M. structor males. They cannot make workers using sperm from their own species. As a result, the workers carry hybrid genomes - one allele from the queen, one from M. structor - but all ants in an M. ibericus colony still have M. ibericus mitochondrial DNA.
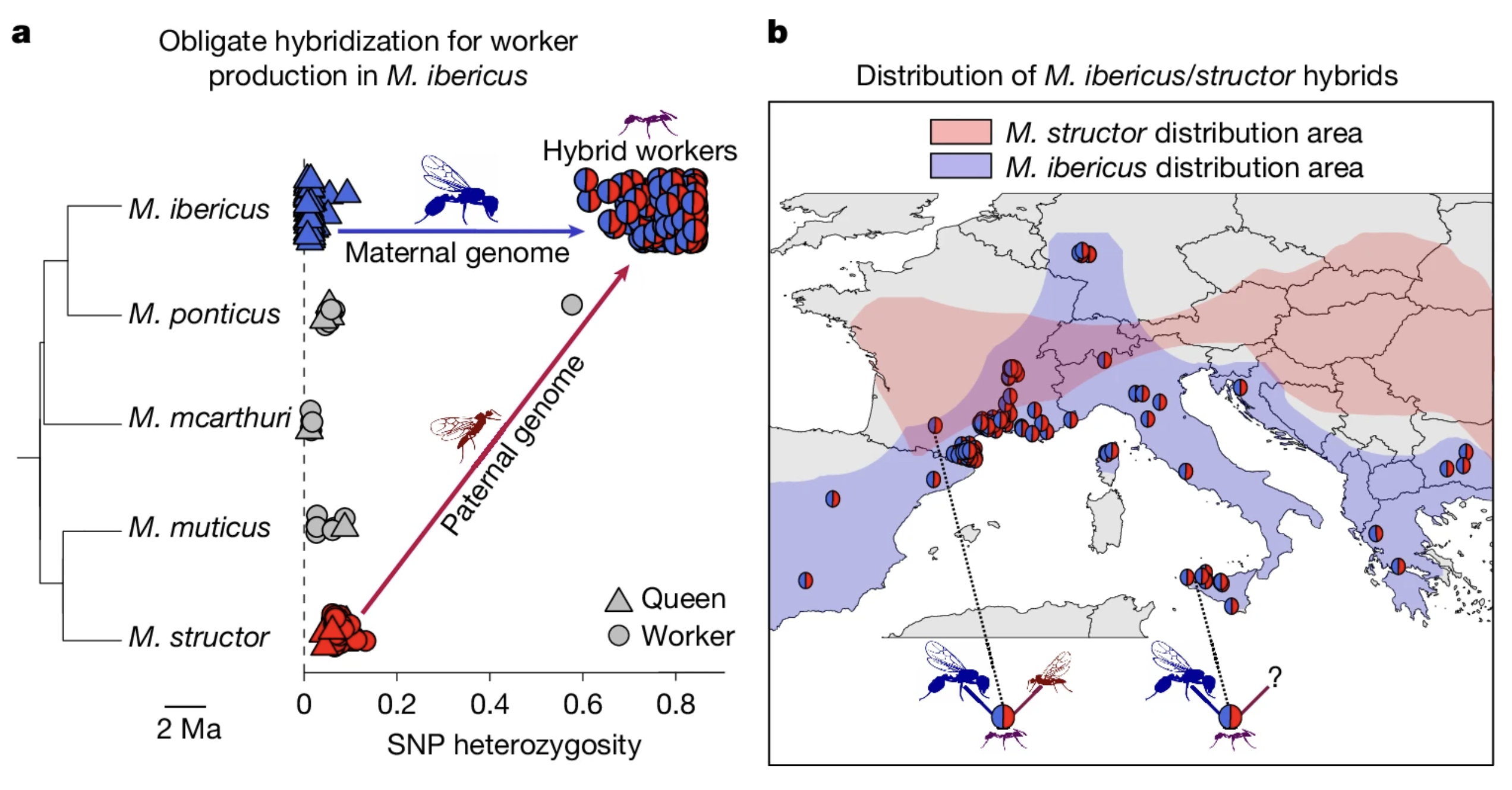
So, it would seem that M. ibericus must always depend on the presence of nearby M. structor colonies, right? Wrong! Figure 1b shows that their ranges only overlap in a narrow stretch. In places like southern Greece or Italy, there are no M. structor colonies at all. So where are the males coming from?
Well, it turns out, that queens can produce haploid M. structor males themselves!
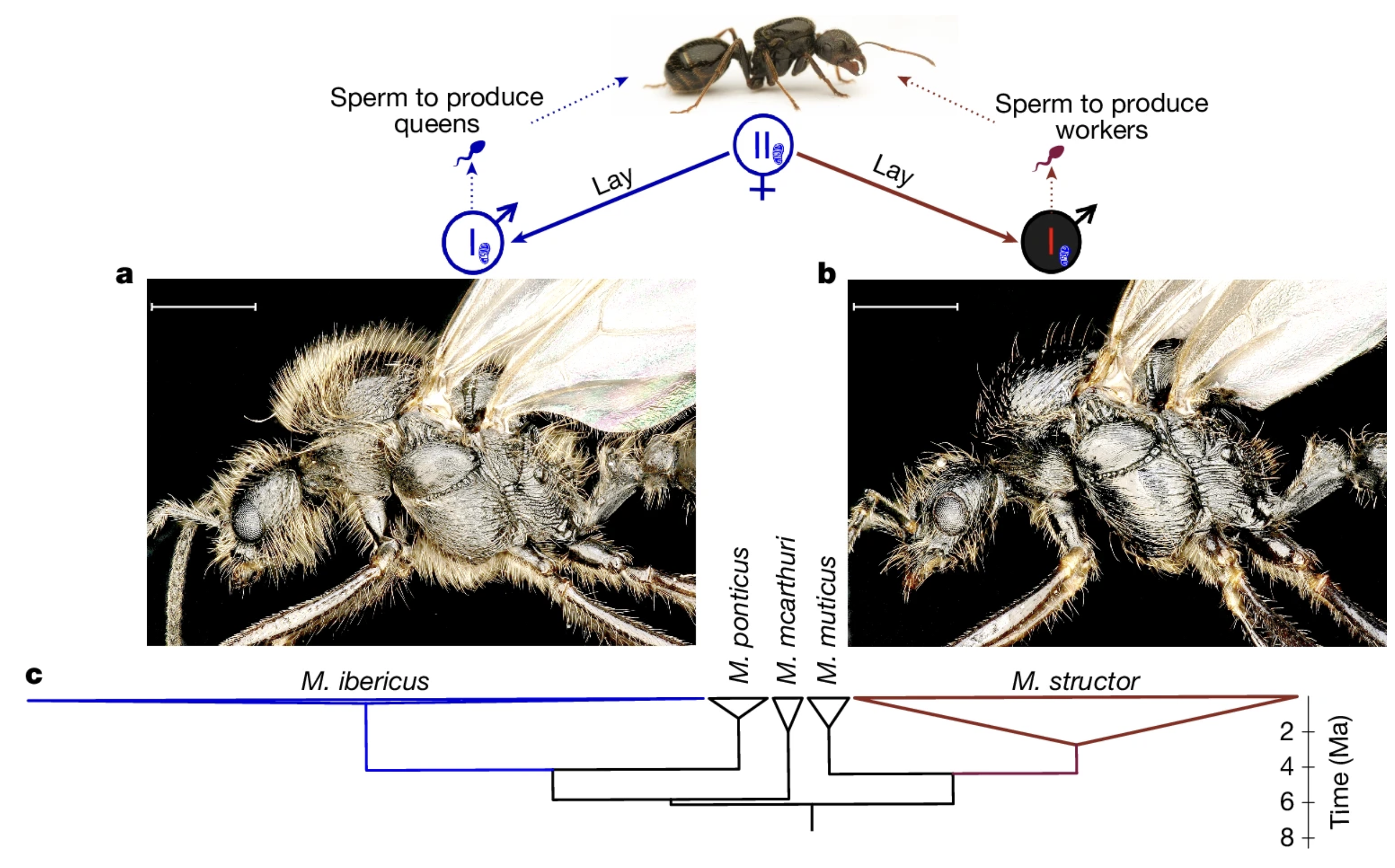
When we get to the “but how is that even possible?” part, we hit the real shocker. After a single mating event with an M. structor male, the queen stores sperm in her spermatheca and starts doing nothing less than cloning M. structor. In these offspring, the maternal chromosomes (except for mtDNA) are completely absent and the zygotic genome comes only from the father.
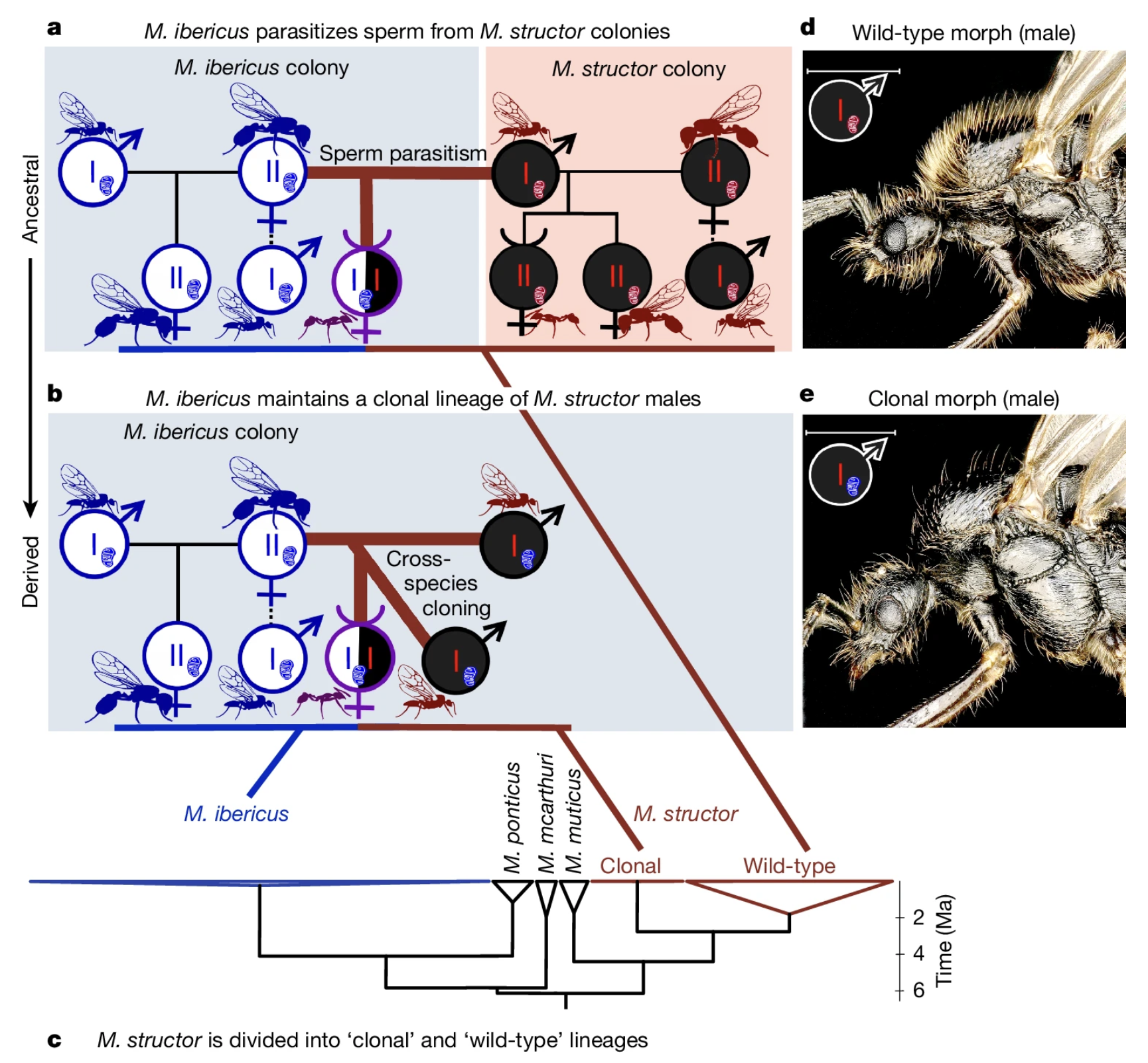
The phenomenon of paternal-only inheritance, also called androgenesis, is not unprecedented; it happens in several organisms and is usually considered a form of male parasitism, since the female gains nothing (look at for example, this study). But in this case, M. ibericus gains everything! It relies on cloning M. structor males as a survival strategy. The authors call this strategy xenoparous reproduction, meaning that one species produces individuals of another species.
References
Vellutini, B.C., Cuenca, M.B., Krishna, A. et al. Patterned invagination prevents mechanical instability during gastrulation. Nature (2025). https://doi.org/10.1038/s41586-025-09480-3
Dey, B., Kaul, V., Kale, G. et al. Divergent evolutionary strategies pre-empt tissue collision in gastrulation. Nature (2025). https://doi.org/10.1038/s41586-025-09447-4
Juvé, Y., Lutrat, C., Ha, A. et al. One mother for two species via obligate cross-species cloning in ants. Nature (2025). https://doi.org/10.1038/s41586-025-09425-w